Differences in replication rates of SARS-CoV-2 variants of concern
Published: 24 February 2022
Research led by Centre for Virus Research and University of Oxford scientists, in collaboration with UKHSA and Brighton NHS, has revealed new insights into transmissibility of SARS-CoV-2 variants and susceptibility to infection.
Research led by Centre for Virus Research and University of Oxford scientists, in collaboration with UKHSA and Brighton NHS, has revealed new insights into transmissibility of SARS-CoV-2 variants and susceptibility to infection.
RNA, a central molecule to the SARS-CoV-2 life cycle, encodes the genetic information required to generate new viral proteins and infectious particles. Therefore, it is vital to understand how SARS-CoV-2 RNA multiplies in infected cells.
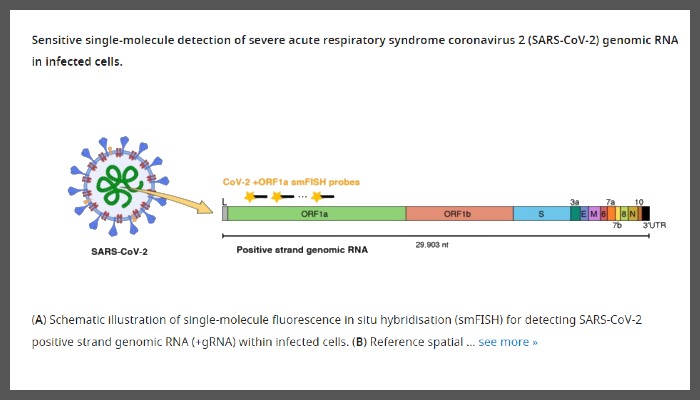
This new study, published in eLife, has developed a new approach to quantify each individual molecule of SARS-CoV-2 RNA within infected cells with greater precision and sensitivity than previously possible.
The approach allowed the researchers to study the very first stages of infection and to estimate the speed in which different variants of concern of SARS-CoV-2 replicates in cells in culture.
SARS-CoV-2, the virus that causes COVID-19, was identified at the end of 2019 in the city of Wuhan. Since then, the virus has spread across all continents causing a pandemic with millions of deaths and leading to a global health and socioeconomic crisis.
Thanks to the extensive effort of researchers and health workers, our knowledge on this pathogen accumulated at unprecedented rate, leading to successful vaccine programmes that have reduced the burden of COVID-19. However, the emergence of variants with increased infectivity and potential to evade protective antibodies produced by vaccination, is a major current concern.
The study showed that the alpha variant, detected months ago in the UK, has a slower replication kinetics than the original virus, suggesting that SARS-CoV-2 variants of concern might not only differ in their ability to enter the cells, but also in the speed in which they multiply their RNA.
The CVR's Dr Alfredo Castello said: "Most of the attention has been centred on the mutations detected in the virus spike protein, as they can influence the ability of the virus to enter the cell or to escape vaccine induced or natural antibody responses.
"However, we should not ignore that there are many other mutations occurring in other viral proteins that can influence virus growth and transmission."
This study pioneered the use of single molecule analysis to study SARS-CoV-2 replication in cells. Applying this approach to other variants of concern such as OMICRON will shed light into their replication dynamics and how these may affect clinical outcomes.
This study pioneered the use of single molecule analysis to study SARS-CoV-2 replication in cells. Applying this approach to other variants of concern such as OMICRON will shed light into their replication dynamics and how these may affect clinical outcomes.
Professor Ilan Davis said: “Most of the available techniques to study viral replication rely on studies of cell populations, providing data that is an average between all cells within the culture or tissue. While useful, it does not allow us to see differences between cells or to study the SARS-CoV-2 life cycle with enough detail.
"Our approach surpasses these ‘in bulk’ techniques because it provides accurate information for each individual cell from the very first time point of infection to the point at which the cell is completely overwhelmed with viral RNA.
"We were also able to show that the technique works in sections from infected lungs of animals, which opens up possibilities in the future to study samples from infected patients.”
Thanks to this technical tour-the-force and close cooperation between a diverse team of researchers, this study revealed that SARS-CoV-2 infection varies across cells, with the majority of cells showing low levels of viral RNAs. However, in a minority (10 per cent) of cells contained extremely high levels of virus RNA.
Jeff Lee, co-author of the study, said: “This level of cell heterogeneity is surprising because these cells were clones. In other words, they are in principle near-identical. “I would expect similar or greater variation between cells in complex tissues, which are heterogenous and contain many different cell types.”
Peter Wing, another co-author, said: “The high variation in SARS-CoV-2 replication in clonal cell lines implies that the cellular state, such as the cell cycle or the response to stress, may define the speed in which the virus replicates.
"It would be of great therapeutic interest to discover the determinants of cell susceptibility to infection.”
The study also revealed that SARS-CoV-2 RNAs are long-lived within infected cells.
Professor Jane McKeating said: “When we treated the cells with remdesivir to inhibit viral replication, we observed that the viral RNA persisted for a long time, being detectable for many hours after the treatment.
“This could explain why SARS-CoV-2 RNA can be detected in some patients long after the clinical symptoms have resolved.”
The paper, ‘Absolute quantitation of individual SARS-CoV-2 RNA molecules provides a new paradigm for infection dynamics and variant differences’ is published in eLife. The work was funded by the Medical Research Council (MRC) and Wellcome Trust.
Absolute quantitation of individual SARS-CoV-2 RNA molecules provides a new paradigm for infection dynamics and variant differences
- Jeffrey Y Lee
- eLife 2022;11:e74153 DOI: 10.7554/eLife.74153
Image Legend: Sensitive single-molecule detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genomic RNA in infected cells. (A) Schematic illustration of single-molecule fluorescence in situ hybridisation (smFISH) for detecting SARS-CoV-2 positive strand genomic RNA (+gRNA) within infected cells.
Funding: The work was funded by the Medical Research Council (MRC) and the Wellcome Trust.
Contacts: For more information, contact Elizabeth McMeekin or Ali Howard in the University of Glasgow Communications and Public Affairs Office on 0141 330 4831 or 0141 330 6557; or email Elizabeth.mcmeekin@glasgow.ac.uk or ali.howard@glasgow.ac.uk
First published: 24 February 2022

