Inflammation-driving SPP1 myeloid pathway could be responsible for post-COVID syndrome
Published: 9 July 2021
Research led by RACE's Dr Mariola Kurowska-Stolarska in collaboration with Professor Elisa Gremese, of the Fondazione A.Gemelli IRCCS in Italy, has found that severe COVID-19 and Rheumatoid Arthritis (RA) share disease mechanisms.
Research led by RACE's Dr Mariola Kurowska-Stolarska in collaboration with Professor Elisa Gremese, of the Fondazione A.Gemelli IRCCS in Italy, has found that severe COVID-19 and Rheumatoid Arthritis (RA) share disease mechanisms.
The study's findings – published in JCI Insight - could help development of new treatment strategies for severe COVID-19 and post-COVID-19 syndrome, or Long Covid.
A previous observation that RA patients who became infected with SARS-CoV-2 had increased risk of ‘flares’ of joint pain and inflammation, hinted at the possibility of a shared mechanism of disease.
Through an international collaboration of both scientists and clinicians, a study focused on the role of cells called macrophages in the link between joint inflammation and severe response to SARS-CoV-2 infection was formed.
Macrophages are immune-cells responsible for engulfing and destroying pathogens and dying cells, which are notorious for their involvement in driving both RA and severe COVID-19 disease.
Using a technique known as single cell profiling, the study investigated the gene activation profile of individual macrophage cells, allowing the identification of a network of distinct macrophage families or ‘clusters’ with diverse functions within tissue.
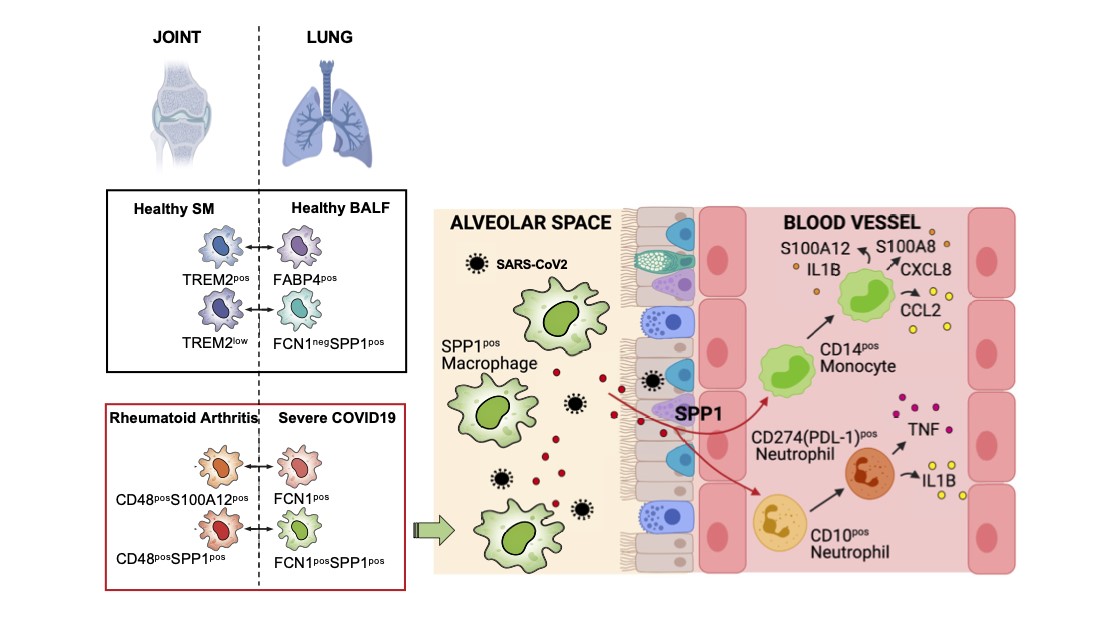
By combining publicly available datasets to compare active RA with severe COVID-19, researchers identified a common population of pro-inflammatory macrophages with shared expression the SPP1 gene, which encodes a protein named Osteopontin (SPP1). This protein is known to be found in bone, but its role in inflammation remained unclear.
To investigate the function of this protein, they stimulated healthy blood immune cells - known as PBMCs - and performed further single cell profiling.
Comparing the SPP1-driven gene activation state of stimulated PBMCs with those found in blood of severe COVID-19 patients allowed the discovery of the effects of severe COVID-19 disease dependent on macrophage SPP1 production and revealed that SPP1 drives a powerful inflammatory response characterising severe COVID-19.
It was found that high concentrations of SPP1 protein could predict whether severe COVID-19 patients required intensive care unit transfer.
Following recovery from disease, the blood levels of most inflammatory mediators resolve; however, SPP1 is maintained at high levels in those with post COVID-19 symptoms.
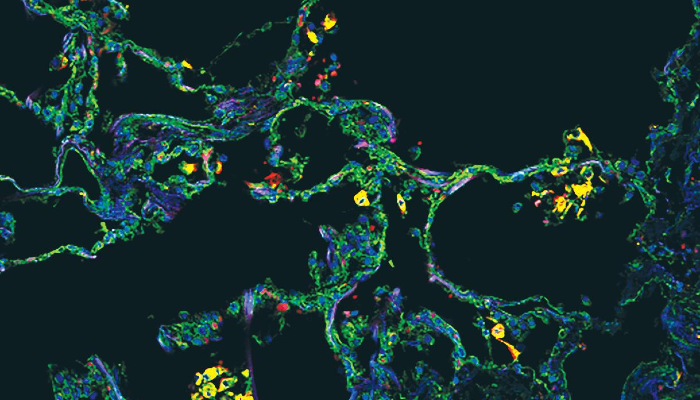
Finally, imaging of the lung found that these SPP1-expressing macrophages are uniquely present in the lung alveoli (or air sacs) of severe COVID-19 in comparison with other types of pneumonia.
This suggests that its proinflammatory function might contribute to long COVID-19, and if so, this identifies osteopontin (SPP1) as a potential therapeutic target for the increasingly common post-COVID19 syndrome.
Dr Kurowska-Stolarska said: “Our investigation is promising, because understanding these mechanisms which drives features of COVID-19 can help open the prospect for new treatment strategies for severe COVID-19.
“Our study findings also suggest that SPP1 pathogenic function might contribute to long COVID-19, and if so, this identifies SPP1 as a potential therapeutic target for this increasingly common syndrome.”
RACE PhD student Lucy MacDonald, a first lead author of this study, said: “We were curious about the most likely common link between joint inflammation and severe response to SARS-CoV-2 infection, which then became the focus of our investigation.
"By understanding this commonality, we have now identified SPP1 as a potential therapeutic target.
"Our goal now is to identify how SPP1-positive macrophages and their mediators may be involved in the long-COVID-19 symptom spectrum, for example musculoskeletal pain.
"Our ultimate aim is to improve the treatment for patients with COVID-19 and post-COVID-19 as well as for our RA patients.”
COVID-19 and RA share an SPP1 myeloid pathway that drives PD-L1+ neutrophils and CD14+ monocytes
- Lucy MacDonald, Stefano Alivernini, Barbara Tolusso, Aziza Elmesmari, Domenico Somma, Simone Perniola, Annamaria Paglionico, Luca Petricca, Silvia L. Bosello, Angelo Carfì, Michela Sali, Egidio Stigliano, Antonella Cingolani, Rita Murri, Vincenzo Arena, Massimo Fantoni, Massimo Antonelli, Francesco Landi, Francesco Franceschi, Maurizio Sanguinetti, Iain B. McInnes, Charles McSharry, Antonio Gasbarrini, Thomas D. Otto, Mariola Kurowska-Stolarska, and Elisa Gremese.
- JCI Insight. 2021;6(13):e147413. https://doi.org/10.1172/jci.insight.147413
- The study was funded by UKRI- MRC, Versus Arthritis UK, and the Italian Ministry of Health.
Image legend: The image, from the cover of the Juy 2021 edition of JCI Insight, shows SPP1+ macrophages in alveoli in a COVID-19 postmortem lung, with immunostaining for SPP1 (green) and the macrophage marker CD68 (red). Nuclei were stained with DAPI (blue).
First published: 9 July 2021

