Chemokine profiling can be used to predict therapeutic potential of MSCs
Published: 17 May 2021
A review article by Institute scientists has concluded that chemokine profiling can be used to predict the in vivo therapeutic potential of mesenchymal stromal cells (MSCs) for diseases such as arthritis, diabetes, transplantation, and Alzheimer’s.
A review article by Institute scientists has concluded that chemokine profiling can be used to predict the in vivo therapeutic potential of mesenchymal stromal cells (MSCs) for diseases such as arthritis, diabetes, transplantation, and Alzheimer’s.
Published in the Journal of Translational Medicine, the paper was completed by first author Nerea Cuesta-Gomez as part of her PhD alongside co-authors Professor Gerry Graham and Professor John Campbell.
Their aim was to examine the potential of MSCs isolated from different tissues to understand the role that tissue source might have in the therapeutic potential of MSCs within a clinical context.
Inflammation is a critical response of our bodies to prevent damage from external agents like bacteria and viruses but also to control and repair injury.
However, errors in the response to inflammation can result in chronic inflammation, where the inflammation becomes self-perpetuating, and cause of disability and mortality worldwide.
Fifty per cent of all deaths are associated with out-of-control inflammatory response-associated diseases, including ischemic heart disease, cancer, stroke, chronic kidney disease, non-alcoholic fatty liver disease, and auto-immune and neurodegenerative conditions like diabetes mellitus or multiple sclerosis.
In order to achieve the aim of regulating excessive inflammation, scientist have been studying the use of mesenchymal stromal cells (MSCs) - a type of stem cell - as cellular therapeutics.
MSCs are so important because they are adult stem cells that have the potential to self-renew and generate several kinds of specialised cells, including adipocytes (fat), chondrocytes (cartilage) and osteocytes (bone).
For this reason, they were originally considered candidates for tissue reconstruction or regeneration, including musculoskeletal tissues, nervous system, liver and skin among others. However, their regeneration potential has only been clinically proven to be effective in MSC-based bone regeneration.
Alternatively, MSCs secrete a plethora of chemokines, anti-inflammatory and immunomodulatory factors, that enable them to modulate immune responses as well as enhance angiogenesis - the formation of new blood vessels - and, thus, are ideal candidates for the treatment of inflammatory disorders.
Chemokines are a group of proteins secreted by cells of the immune system and MSCs that act as chemical messengers to guide cells to sites of inflammation.
Through the secretion of distinctive chemokines, MSCs can recruit specific immune cell populations.
Through the expression of distinctive chemokine receptors, MSCs are able to migrate towards specific tissues based on the chemokines present in their surroundings.
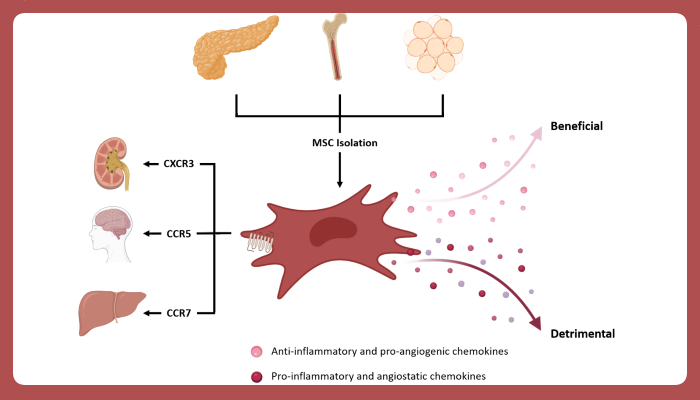
MSCs can be isolated from most tissues around the body, including umbilical cord, cord blood, placenta, dental pulp, periodontal ligament, adipose tissue, and pancreatic islets.
However, based on the tissue source of isolation, MSCs express different chemokine receptors and secrete different chemokines, anti-inflammatory and immunomodulatory factors.
Therefore, and as previously mentioned, the aim of this paper was to discuss the differential migratory, angiogenic and immunomodulatory potential of MSCs isolated from different tissues to understand the role that tissue source might have in the therapeutic potential of MSCs within a clinical context.
In this review article, the authors describe how the recruitment and homing of MSCs to sites of injury is essential to contribute to tissue repair, revascularisation and regeneration, as well as to dampen inflammation and avoid the activation of the immune system.
As MSCs isolated from different tissues express different chemokine receptors, tissue source of isolation could dictate MSCs migration potential and thus, understanding the role of specific chemokine receptors in relation to migration towards specific anatomical locations could make MSCs isolated from some tissue sources more desirable than others for specific clinical settings.
Furthermore, they state that chemokine profiling enables the prediction of immune cell recruitment, immunomodulatory potential and T cell inhibition potential.
They conclude that profiling of chemokine receptors, as well as chemokines, anti-inflammatory and immunomodulatory factors secreted by MSCs would enable to identify the most appropriate source of MSCs to use as cellular therapeutics for different diseases, including autoimmune diseases like arthritis and diabetes, transplantation, or Alzheimer’s.
Nerea Cuesta-Gomez said: "Diabetes, Alzheimer’s, Parkinson’s, arthritis, and cancer, to mention a few, are diseases that touch every family in the world, and they have the potential to be cured through stem cell research, or at least, to improve the quality of life of those affected by them and their families."
Chemokines and their receptors: predictors of the therapeutic potential of mesenchymal stromal cells
Image legend:Figure 1. Chemokine receptor expression and chemokine secretion predict therapeutic potential. MSCs isolated from different tissues have a differential chemokine receptor expression and chemokine secretion that results in differential potential as cellular therapeutics.
First published: 17 May 2021

