AdhE transition forms extended spirosomes
Published: 19 June 2020
Research involving iii's Professor Andrew Roe has provided a mechanistic understanding of the regulation mechanisms of Aldehyde-alcohol dehydrogenase activity via structural transition, and a platform to modulate its activity for developing antibiotics and facilitating biofuel production.
Research involving an Institute scientist has provided a mechanistic understanding of the regulation mechanisms of Aldehyde-alcohol dehydrogenase (AdhE) activity via structural transition - and a platform to modulate its activity for developing antibiotics and facilitating biofuel production.
The collaboration between iii's Professor Andrew Roe, the School of Life Sciences (SoLS)' Professor Olwyn Byron, and colleagues at the Korea Advanced Institute of Science and Technology, led by Dr Ji-Jong Soon, was recently published in Nature Communications Biology.
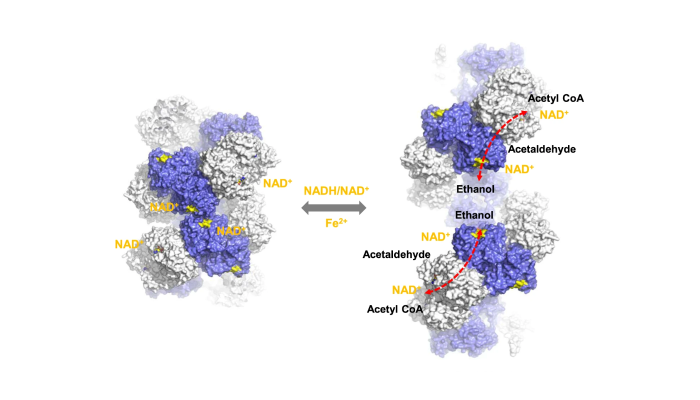
Their work follows on from a previous paper, published in Nature Communications last year, in which the team reported, for the first time, the atomic structure of AdhE, an enzyme from the bacterium E. coli.
This protein is interesting because it assembles into large superstructures called spirosomes that form compartments within bacterial cells.
In the latest paper, the team used cryo electron microscopy to see how the spirosomes change their shape when they bind a cofactor.
This allows AdhE to channel substrates through the centre of the spirosomes, maximising enzyme efficiency.
For pathogenic strains of E. coli, the University of Glasgow group have found that the enzyme is important during the infection process, probably because it is so central to energy generation in the bugs - this makes it a target for the development of anti-virulence drugs.
At the same time, AdhE is of importance for the biofuel industry because it is involved in ethanol production.
Andrew Roe, iii Professor of Molecular Microbiology, commented: “AdhE spirosomes are like mini-factories producing ethanol in a really efficient process.
"Exploiting how bacteria produce ethanol could help in the biofuel industry and enable the move away from gasoline powered vehicles."
Olwyn Byron, SoLS Professor of Biophysics, added: “This latest paper will transform our ability to take this work forwards to develop specific inhibitors that might function against a range of Gram-negative pathogens. We’re excited about getting back to the lab!”
Aldehyde-alcohol dehydrogenase undergoes structural transition to form extended spirosomes for substrate channeling
- Gijeong Kim, Jinsol Yang, Juwon Jang, Jin-Seok Choi, Andrew J. Roe, Olwyn Byron, Chaok Seok & Ji-Joon Song Commun Biol 3, 298 (2020). https://doi.org/10.1038/s42003-020-1030-1
First published: 19 June 2020

